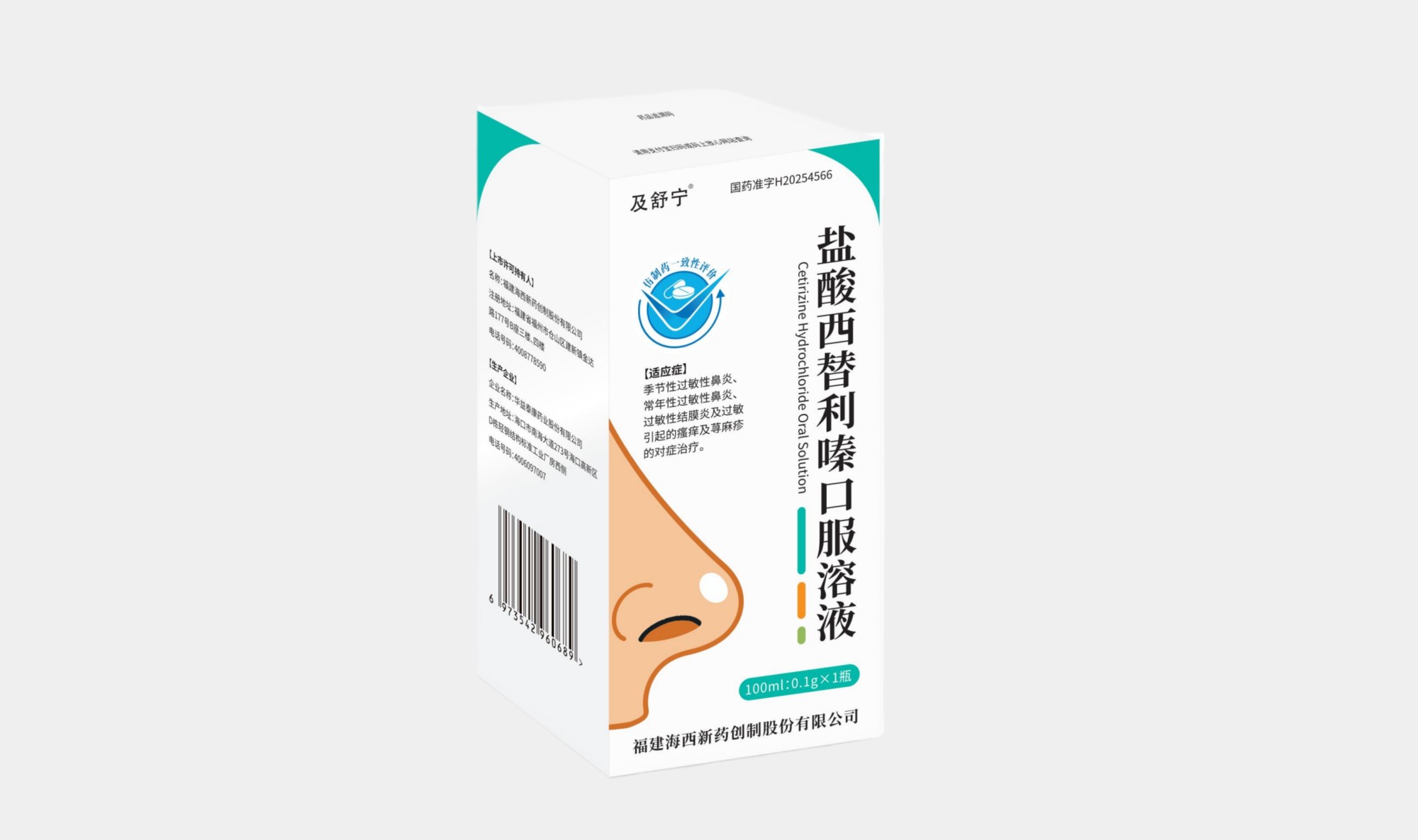
Jishuning® Cetirizine Hydrochloride Oral Solution
2025 Guangdong Alliance: Diclofenac and other drugs to continue the selection.
Medicare Category B
Wide applicability; precise dosage; convenient to take; stable absorption.
[Drug Name]
Generic Name: Cetirizine Hydrochloride Oral Solution
[Indications]
Seasonal allergic rhinitis、Perennial allergic rhinitis、Allergic conjunctivitis、Symptomatic treatment of pruritus and urticaria caused by allergies
[Usage and Dosage]
Recommended for adults and children over 2 years old.
Adults and children over 6 years: In most cases, the recommended dose is 10 ml (10 mg) once a day, taken orally. If the patient is more sensitive to adverse reactions, the dose may be split, with 5 ml (5 mg) taken twice a day—once in the morning and once in the evening.
Children aged 2-6 years: 5 ml (5 mg) once daily; or 2.5 ml (2.5 mg) twice daily. (See the product insert for further details.)
[Contraindications]
Contraindicated in patients who are allergic to levocetirizine, cetirizine, other piperazine derivatives, or any excipients of this product.
Contraindicated in patients with end-stage renal disease (CLCR < 10 ml/min) and patients on dialysis.
Contraindicated in children under 11 years old with renal impairment.
For further details, please refer to the product insert.
[Specification]
100 ml: 0.1 g
[Validity Period]
24 months. After opening, the product should be consumed within 3 months and should not exceed the expiration date. Discard if expired.
[Approval Number]
National Medicine Approval Number H20254566
Consultation hotline: +86 591 83512959